topic Biological contamination of the filters, Published in: Das Schwimmbad und sein Personal, edition 09/2018
... or what is possible to do about the risk areas in pools and water treatment.
With a water treatment and disinfection after the letters of DIN everything is done? By no means!
Where does the air from your air flushing? If you do not know it or you can smell during the flushing, then you should urgently and carefully read to the end.
Again, this post assumes, that flocculation and filtering are set optimally and work. The hints, which the Federal Environmental Agency in the notice: "Hygiene requirements for pools and monitoring" 2014 is about the quality of filtering given, we had in a previous text in the October 2016 implemented.
The restriction to letters DIN feel, who fighting in their objects against biological conatmination of the filters or "infectious" whirlpools. The risk areas are in DIN 19643 mentioned, But as so often come to my mind causes, relationships and effective measures too short. Admittedly, now I have to create some chaos. But chaos is not mine, but by the many committees, write the rules.
Example? DIN 19643-1:2012-11, Table 7: If in the initial examination Legionella in the pool water between 1 and 100 CFU/100ml be found , then follows the measure "follow-up". Upps, the investigation takes using traditional methods in the laboratory 10 to 15 days.
But the Legionella are much faster than the German regulations. I have read, these things are doubled under optimal conditions all 2 hours (Langer 2012), but we better stay with conservative 6 hours for a doubling, otherwise the numbers are too large. In the 13 days between sample and laboratory result elapse 312 hours, if the "happy event" all 6 hours enters, these are 52 duplications. Let's start with one CFU we are mathematically so after 13 Days theoretically a stock of 252. The computational results are 4,5 quadrillion (comes immediately after trillion, 15 zeros).
Error analysis
I would say, the result of the laboratory study can NOTHING declare about the current biological state in the system testify. The day of sampling may decide, if 99 "acceptable" KBE be found or 24h later on 994 = 96 Million CFU the pool must be closed. The cooling of the sample during transport can distort the result. Just as remnants of the disinfectant can act on the sample and lulled into security, that does not exist. Of course, the theoretical considerations are rather unrealistic, because the Legionella also opponents in your biological environment. Nevertheless, we must be clear that. For the next lines especially.
Orientation to the DIN
According to DIN what follows the follow-up in the unfinished example? You guessed it certainly, a follow-up! And an additional control of the filtrate.
If the values it really unexpected already on the line 100 to 1000 CFU/100ml have changed, then among other follow-up following measures are called. Quote: "Further actions involving professionals, for example superchlorination, replacement of the filter material… switch off the aerosol-producing devices; Notification to the relevant health authority, repeated follow-up of pool water and filtrate. "
If we were to pursue the timeline, of course, we also need to schedule of the imaginary professionals (Pay attention, plural!) consider. But we know in common, what is meant and appreciate the good intention of securing contracts.
So we follow the hints, which are hidden in the Quote. The first is to make "control of the filtrate" and "replacement of the filter material". If found in pool water Legionella, then it should also be monitored in the filtrate according to DIN. Filtrate is by definition: "Filtered water before any aftertreatment and front of interference of the disinfectant". Of course, we should ask ourselves, why in the DIN not the filter is named as a possible source. Especially, if the water is by definition directly from the filter.
CFU/100ml
At least here we have to take care of us first terms. The unit "CFU/100ml" means, that in the laboratory from a 100ml sample of water visible groups of bacteria, so-called Colony Forming Units, cultivate and let COUNT. These grow after transmission on a particular nutrient, at a certain temperature in the incubator in a certain time.
Legionella
... are a genus of rod-shaped bacteria of the family Legionellaceae. They are waterliving mobile bacteria. Legionella are hazardous to health for people. At present we know more than 48 species and 70 variations. The most important for human disease species is Legionella pneumophila, it is causative agent of legionellosis or Legionnaires' disease. Here is a real recording of the electron microscope:
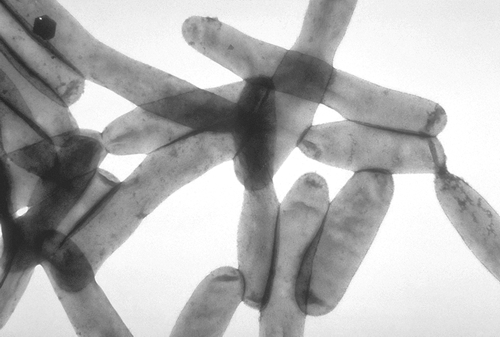
Photo 1: Legionella, Source: By CDC (PHIL #1187) (CDC Public Health Image Library.) [Public domain], via Wikimedia Commons
To understand the effect, we must also briefly with the
Legionnaires' disease
reflect. Legionnaires' disease was 1976 described for the first time. It was named after a meeting of US war veterans association American Legion, the from 21. to 24. July 1976 held at the Bellevue-Stratford Hotel in Philadelphia. At the time they were 181 older people suffering from pneumonia life-threatening. All were either visitors of the war veterans meeting or visitors of the hotel. The legionella had been able to settle in the neglected air conditioning in the hotel.
People had taken the legionella by inhaling infected aerosols. Where to find? In the whirlpools, over the floor bubbler and all other air attractions. But for the staff of the technic, there is a more dangerous source: The air from the filter flushing (with air). Where does the air in your filter room go??
The first dilemma
As simple as desired, does not make us the legionella. To a flat bacteriologist joke:
The legionella sits and listens,
rushes as chlorine past her.
ha-ha laughs ironically,
I live, is not that funny?!
Not only people often dodge in the way of the nutrition. Even in the kingdom of amorphous creatures (and we're not talking about unfaithful husbands, but from protozoa under water organisms) this error occurs. (To all wives of lifeguards: The resulting joke is even flatter than my predecessor!)
We're talking particularly of "amoeba", the Legionella stands on the wet menu. While the amoeba an involuntary but good shield against chlorine and other anti-Legionella intentions is, the Legionella can proliferate under this protection. Similar to a parasite benefits the Legionella even from the dead amoeba, because the chlorine can not distinguish between living and dead organisms.
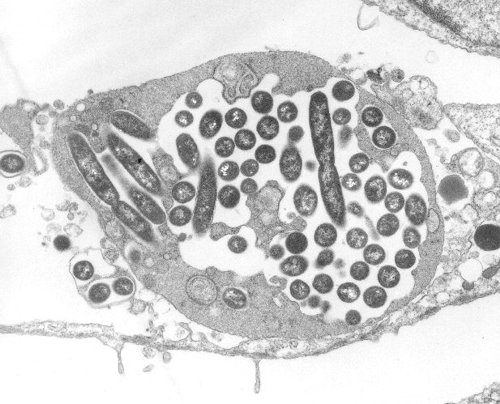
Photo 2: Legionella in Amoeba, Source: wikipedia.org
Biofilm
Healthy water is NOT dead water! Organisms live in it, thereby improving water quality. It may not be too many only.
We humans support this increase with nutrients from the materials of the pipes, with nutrients from poor filtering and advantageous high temperatures. These organisms then deposited in pipes and form a biofilm. Such a biofilm as an insulator for the underlying layers. What's inside again, is hard to remove.
Metaphorically speaking: Now the dead but well-stocked Amoeba hangs in the biofilm, will not find their way into the sample vessel and fills the stocks of Legionella after about. 4 days fully back on.
Photo 3: Illustration Biofilm, Source: Fotolia
The second dilemma!
Let's return to the presumption, that the Legionella originate from the filter. The manufacturer of a filter is to be trusted, that his material does not transfer substances to feed the organisms. Trust is good, Control is better: There was a KSW (plastics in the pool water) directive to study the plastics in exactly this assumption. The results of this study can be show by renowned manufacturers for the used interior material.
So if legionella in the filter sit, then in the filter material and not on a platter. Rather well hidden in a living amoeba, with pleasant temperatures and possibly also on huge the surface of activated carbon. An ideal habitat and probably free of disinfectants.
We operate the swimming pool with ever lower concentrations of chlorine. In the pool are in the maximum case, 0.6 mg/l to find. The free fall over the edge into the (almost empty, because oversized) gutter drain removes another reminder of the chlorine concentration. Then it could additional be, that foliage has accumulated in the expansion tank, again is part away. When the ballance tank is then also build by pure concrete, goodbye further chlorine. If we're lucky, then additional will iron- or manganese-containing filler water added. The oxidation reaction then eats almost all traces of the free chlorine. We do not know all that sure, because measuring the concentration of chlorine is in the pool and not before the filter.
Activated carbon against bound chlorine
... or even against the free? If someone had the glorious idea now, to use activated carbon in the filter, then the water is approaching a status as fertilizer after the first few centimeters.
To repeat, Activated carbon is characterized by an extremely large surface. 4 grams of it, offer the size of a football field.
A surface for attachment not only to the chlorine molecules but also a home for the biofilm. So if activated carbon in the filter, whether applied powder coal or permanent granulocarbon, then the activated carbon protects the biofilm in the lower layers. Legionella can multiply.
The countermeasures according to DIN
The further notes from DIN I would not exactly call helpful. Superchlorination? To annoy the amoebas and to feed the legionella? Chlorine in the backwash water? The same effect! And who has the time, Filters for at least 24 hours to lay down? (Hygiene requirements for pools and their monitoring, 2014, Paragraph 5.3) Who has planned a reserve filter in order to be able to supply the standardized volume flow within the "superchlorination"? Or just the replacement of the entire material? ... in order to 20 days to get the problems again?
No, this approach should be reconsidered!
UV light
... changed the DNA of the Legionella, and prevents them from multiplying. Side reactions can even kill the Legionella. But how do you explain a Legionella, that they should move from the cozy filter bed to the lamp? And if we succeed with that, Disinfection means, by definition,: It stays 1 from 10000 Legionella unscathed. Of course it is possible, to reduce the numbers of "active", "viable" Legionella in the pool water temporarily.
But the cause is not combated with it. In my view, no solution too. So we need to bring an effective disinfectant to Legionella, possibly past the protective shield of the activated carbon. What does the DIN 19643 say to this?
Chlorination of the backwash water!
The first approach to this is, of course,, to make the backwash water chlorinated. But as in any of the previous article already discussed, reacts the chlorine first with known mineral and organic substances. Whether then something is left to kill, or can actually penetrate the amoeba, is questionable. Especially since our water saving motivates us to do so, to keep the so-called "contact time" extremely low. Meant is the time, that the chlorinated water has to "contact" the organism, until an effect can be seen.
Photo 4: Illustration chlorine in the periodic table, Source: Fotolia
A little confuse me the concentrations specified for the chlorine. My personal favorite quote from DIN 19643 is in the section 4: "For disinfecting, a germ killing of Pseudomonas aeruginosa by four powers auf ten was within 30 s based. "
Translated this means, the concentration of 0.6 mg/l chlorine reduces the numbers of Pseudomonas aeruginosa to 99,99%. Whether these numers are correct, we want to do not doubt, but put into context with other values.
The resultant "concentration-contact time-product" is 0.6 mg/l (for maximum) times 0,5 min, so 0,3mg/l*min.
So this value applies to the pool, where the dirt is entered and the people should be protected. We know: If you find something in the pool, you should look in the filter. If you find something in the filter, you should do a "superchlorination" ... Luckily, the Federal Environmental Agency has this term in paragraph 5.2 underlaid with values. For the same disinfectant chlorine in the filter is for the ...
Superchlorination
called for a concentration-contact time-product of 10mg/l times 120min = 1200 mg/l*min! This is more than 4000 times!
If Legionella were found in the filter, then the Federal Environment Agency increases this value in paragraph 5.3 for chlorine to 36000 mg/l*min (in words: thirty-six-thousand, 50mg/lx12h). Alone between the "standard germs" and Legionella is an increase to 9 times! Legionella must probably be extremely dangerous organisms. One reason could be the research results of Kilvington and Price1990. There is paraphrased: The cell wall of the amoeba is relatively resistant to chlorine, so that trapped Legionella survive chlorine concentrations of up to 50mg/l.
But what's confusing?
Answer: The whirlpool!
Because the temperatures in the hot whirlpool and the circuit pipes are an ideal place for biofilm and legionella. In the moment of bubbling, the Legionella are transported directly into the breathing air of the guests. That could be the reason, why in DIN 19643 the chlorine concentration 0,7 was increased to 1.0mg/l. The corresponding concentration-contact time-product is only 0.5mg/l*min, only 1.6 times. Are Legionella in a hot whirlpool younger or "thin-skinned" as in the filter? To excuse we have to see, there are several years between the two documents.
But could the values be based on the principle "Much helps a lot!“?
Small interim remark: Of course, the constantly flowing water pipes are not the legionella oasis to be looked for. Air pipes without connection to the disinfectant of pure water do not exist theoretically, but in practice nevertheless ...
Again back to activated carbon
Consciously, a part has ended with a hidden reference to an "effective disinfectant". This means, Chlorine is ineffective in the vicinity of activated carbon? Yes, because if the activated carbon purposely removes the bound chlorine, then the free chlorine shares unintentionally the same fate.
Ok, you can send so much chlorine into the filter, that the surface of the activated carbon is too small for all of the free chlorine molecules. The surface is occupied but after the action, the activated carbon is almost worthless. Then you could replace the activated carbon immediately and economize the chlorine.
Ozone? Is well known, and fortunately with activated carbon in the same opposing relationship. Excess residues are not allowed, so to get from bad to worse. Even faster than the with chlorine!
Effectiveness of chlorine
The ultimate goal: combat legionella! Requirement: Effective disinfectant! Affects the chlorine?
Who remembers the article for disinfecting, has certainly remembered the following diagram:
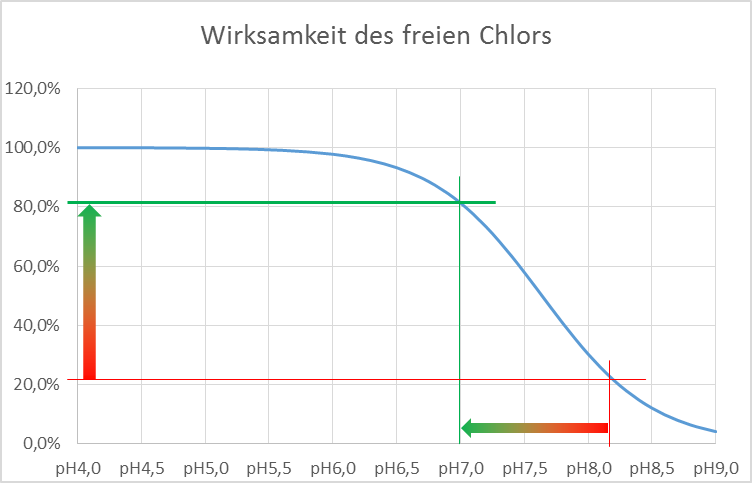
Illustration 5: Representation effectiveness free chlorine
Anyone not remember, the article is available online here or clicking through the publications.
The note, wherein the pH should operate with the chlorine, I could not find in the recommendations of the different documents of the authorities. Presumably, it plays a role, if 100% of the free chlorine are effective or only 10%.
Let's do it that way, so if we follow the recommendations. If one uses to chlorination, the liquid sodium hypochlorite according to DIN 19608 then metered in a liquor with a value of about pH11! The hobby- or professional chemists can calculate, which change the pH at concentrations of 10 50 mg/l has to go through.
By the way, on the side, before it is too late, not forget the resulting lime precipitation in the filter! The lime can then solidify in the sand 24. It is therefore necessary to correct the pH.
In the end, one must also clarify, whereas with this chlorine water. What is not consumed, is probably not welcome in any wastewater treatment plant. What is consumed, this is called now "Bound chlorine" and spread the known intense chlorine smell. Beware overcarefull staff or visitors, who like to deduce a chlorine gas accident from this smell!
This raises the question, why it's always thought about chlorine. Is it because of the interests of authors? Or at the well-trodden paths of the chemical mace ... But we only have to read carefully, to be brought by the Federal Environment Agency to track.
Chlorine dioxide comes into play
Recommendation of the Federal Environment Agency Paragraph 5.3 „When using chlorine dioxide is the minimum concentration of 1.0 mg/l (compared to 10 mg/l chlorine) is too pay attention.“
Is this remedy more effective than chlorine? Of course, one has researched this. Mr. Bozenhardt has 1994 published, that chlorine dioxide on Escherichia Coli about 3-times more effective than chlorine. Why? Well, the first difference is already described with the molecule. In chlorine dioxide ClO2 have 2 Oxygen atoms is 1 coupled with chlorine atom. This is a "strong bond". At least stronger than between the two chlorine atoms of the free chlorine Cl2 (HOCl / OCl–). The urge, to bond by oxidation with other components of the water, is higher then in Free Chlorine. But the free chlorine can differ not "living" to "dead" matter. So it is probably pleased about the iron and manganese in the water and likes to color:

Drinking water after 120 minutes contact with the air, Photo: aqua&pools
The chlorine dioxide already acts as a poison on the living organisms in lower concentrations and penetrates the cell walls. The oxidation effect is lower, but available too. Often forgotten, that itself is approved as a disinfectant in the strict German Drinking Water Ordinance.
A connection to the pH value is only to a lesser extent, Chlorine dioxide is dosed as an acidic solution.
For comparison, Chlorine dioxide in drinking water
A cycle is characterizes the water treatment in the swimming pool. The drinking water is delivered unique and consumed. Nevertheless Legionella occasionally occur. If there were exceeded the 100 KBE/100ml, then the disinfection with chlorine dioxide is recommended. The contact time between dosing and the first sampling point is completely variable. But the maximum concentration of 0.2 mg/l is fixedly placed at the first extraction point. Extreme concentrations of 10mg/l would endanger human.
Purchasing the First:
For producing chlorine dioxide, there are several chemical processes. All should be common, that the result is not dangerous. Is one storage a chlorine dioxide solution like in a washing bowl, then evaporate the chemical. The higher the concentration in the liquid, the higher the concentration is in the air layer above. Smell or not, when a critical level is exceeded, then it explodes! Among chemists' - "tends to explode". Therefore, only very dilute solutions are storable. DVGW (German Association for Gas and Water) guidelines describe procedures, generate usefully concentrations of less than 3 g/l. In times of major search engines one can (unfortunately) buy everything.
Many finished products are advertised with higher concentrations of chlorine dioxide. Can be tricked here chemistry, or the client is the victim? In my experience is exploited, that the DPD-1 measurement can not distinguish between free chlorine and chlorine dioxide. Substituting a Membranbedeckten Sensor on, it quickly becomes clear, that not always chlorine dioxide is in it, where chlorine dioxide is labeled. Who wants with the DPD method verify, he should eliminate the free chlorine with glycine and now measure chlorine dioxide.

Membrane-covered amperometric sensor, Source: aqua&pools
Purchasing the Second:
Of course you can bring the essential components manually to a reaction on the spot and has chlorine dioxide. Pay attention, this is not an invitation! Because the risks of mishandling are huge. Lost water or incorrect shares, already it can crack hard. Who does not believe, should contact in advance with his insurance company.
Purchasing the Third:
At least one manufacturer uses special raw materials for a chemical reaction. Is used as one of the starting materials sodium peroxodisulfate, then the reaction takes place comparatively slowly. Quote: "The patented process behind DK-DOX® enables the safe manual of effective chlorine dioxide water treatment. In the union of the two starting components there is no spontaneous formation of chlorine dioxide. Moreover, explosive concentrations of chlorine dioxide are not generated at any time during the reaction. The easy and safe handling of DK-DOX® is therefore assured at all times. The trade association rated the chlorine dioxide generation system DK-DOX® as harmless, from the perspective of occupational safety. The use of ready-made solution as canisters goods allows the versatile use in different areas." Source: https://www.dk-dox-brau.de/index.php?id=26
Purchasing the Fourth:
In the citation approach to rapid variant infected. The more time the reaction to chlorine dioxide is used for transport. Then chlorine dioxide is only in it, if the container is at the customer side. No low cost solution, but an idea for the absolute emergency.
Purchasing the Fifth:
The safe production of chlorine dioxide can (and should) be left to a generator plant on site. This plant is with electricity, Water, hydrochloric acid and sodium supplied. Pay attention, Sodium chlorite NaClO2 is not known salt sodium chloride. The plant produces a storable chlorine dioxide solution with a maximum of 3g per liter chlorine dioxide. The performance of such investments is to specify in grams per hour, Therefore, it is favorable also to know their own needs in this unit. A tutorial for selecting such generators is here to find.

Photo 5: Chlorine dioxide generator, Source: aqua&pools
The decision, which method of procurement is favorable, of course, also depends on the size of consumption. Therefore, a small guide with an Excel spreadsheet in the tutorials for water disinfection is at www.aquaandpools.de online. But let us at last to the practical implementation:
The biggest mistake
... is the disinfectant dosing in the ongoing stream of water rinse. Quote Federal Environment Agency (without Legionella): 10mg/l of chlorine with a reaction time 2h. Wih Legionella: 50mg/l of chlorine with a reaction time 12h or 10mg/l of chlorine dioxide with an exposure time of 24h!
Who please wants and can its water rinse on 12 hours extend? At 60m/h speed these are 120m³ water per 1m² filter surface. As an an example: 25x10m as a swimmer pool give about. 110m³/h flow rate, and filter area 3,7m². At a filtration rate of 60m/h so 440m³ of water are sent to waste water. The pool must be 1.75m deep, so that the content for a filter cleaning is enough. Joking aside, the volume flow-proportional dosage in the rinse water is nonsensical, therefore, the huge consumption of chlorine or chlorine dioxide does not need to be recalculated.

Photo 6: A taste of chlorine consumption, Source: Fotolia
The logical recommendation
... should be so:
- Perform the normal flushing process without disinfectant, because here the contact time is too short anyway.
- After rinsing with water stop and insert a new step. In preparation should the existing residual water (Volume - filter sand) calculated once or practically test.
- 10mg of chlorine dioxide per liter of water metering in this comparatively small residual volume of water.
- give a very short air-flushing shock (only a few bubbles) so that the chlorine dioxide is distributed in the volume of water and backward reached the appropriate pipeline at the end of the air-purge shock and disinfected.
- Waiting time 24h. Do not scare, see below for the possibility of reducing.
- With the step "First filtrate" resume the process.
For me, the approach is, to give a lot of disinfectant for a long time into the filter, absolutely unsatisfactory. Where does he comes from? In my opinion, from the uncertainty of the experts on the results. This all costs time and money, but in the section "Error analysis" we had to confirm the uncertainty. True to the slogan: "Looked over the plate edge!" we change to question:
What does a sand filter and a cooling tower have in common?
Answer: The ideal living conditions for Legionella and the direct effect of legionella to the humans. Not to forget, the damage replacement claims of the affected people. Also in the industry of water treatment for cooling towers biology must be got under control.. VDI (Association of German Engineers) and Federal Environment Agency raise with rules and regulations for some time the pressure on operators. Key point: All cooling towers are recorded in a register and must be checked by an accredited laboratory. The accredited laboratory is then even obliged, to report Legionella found at the authorithy. The authorithy, in turn, may order the immediate closure.

Photo 7:Cooling tower in Portugal during disinfection
But the problem is similar: The long time between sampling and available results . We change in general industry, we take a brewery as an example. If their water is contaminated with Legionella, then presumably the production must 13 days (since the removal of the sample) be disposed of. The loss would be huge. For relief, I do not know any brewery, which excludes this uncertainty not from the outset through continuous metering of chlorine dioxide.

The panorama shows a brewery in Ethiopia, whose chlorine dioxide plant I put into operation several months before the start of production.
So many operators of cooling towers are currently sitting in front of the interplay between perceived security described above (even more) Laboratory investigations, identified uncertainty of waiting for the results and the risk of plant shutdown.
All three factors could eliminate, if you would have a faster method to determine the concentration of Legionella in a sample. Imagine yourself, you could take a sample and know after 1 hour, how many living Legionella of dangerous subgroups be in the sample. Because these are exactly the characteristics, which are also necessary for the laboratory study. You guessed certainly what's coming: "There's already!“
Mobile Legionella Laboratory measurement
With an hour of concentrated activity and the necessary equipment is known, how many Legionella are in the sample. A description of the function would "swallow" here are a few more pages, Therefore, for all who want to know more: www.aquaandpools.de/innovative-produkte/.
Photo 9: Mobile Legionella Laboratory measurement, Source: aqua&pools
Important to know, that the method of mobile laboratories Legionella is made use of international, may not be used but in Germany by accredited laboratories.
If the lab turns after 13 days have to do with poor results on the way to the authorithys, the owner of a mobile laboratory legionella had almost 2 weeks for countermeasures. Who owns this device or knows, which owns the device, is now ready for the ...
Optimization
... the course of the flushing. For now, you can already watch during disinfection, what has been achieved. The first goal should be reducing the time, so that the filter can fulfill its purpose again. Pay attention, a second attempt for this observation should not exist. Therefore you practice before flushing the measurement at (supposed) germinated filter. The handling of the mobile Legionella laboratories must sit, ideal for two people.
The necessary chemicals cost money, but the possible savings pays for itself easily against it. If the procedure of the evaluation was practiced, takes you 1,5 Hours before the first "real" flush a sample and evaluates. 15 minutes after the dosage of the disinfectant, the first sample is taken and immediately started the evaluation.
At 30 Minute contact time, a second sample is taken and evaluated parallel. For this, the second person is advantageous. After 60 Minutes, the third sample, and then a further sample will be taken hourly.
Presumably it will come not so far. Because with 10mg/l chlorine dioxide Legionella should after 15 minutes have arrived in the realm of dreams. Smaller distances than 15 Minutes are difficult to achieve with one device, because the individual steps of the evaluation mostly take 5 or 10 minutes while. Moreover 15min break in the filter rinsing appear reasonable.
From the time after:
What is happening, if we were successful in filter disinfection? Biology is "done" by the chlorine dioxide, but still in the filter. You could follow up on a second filter flushing, which is to deliver the dead biofilm. But whether nutrients are more or less available, the "sanitized" surfaces will be resettled.
Biological countermeasures
In other systems, could now occupy the space after disinfection with bacteria from the "probiotic group", but they are not tolerated by the ongoing chemical disinfectants our technological DIN-filter-world. On a few details of the Biological displacement I will discuss in a later post, where appropriate, I present the explanation soon online.
The biofilm begins immediately after disinfection with the renewed settlement. How fast that goes, is the lever for ...
Optimization, the Second!
If we take daily samples after disinfection and investigate with the mobile laboratory legionella, then we quickly see the time course of infection. So we can make a prediction, after how many days the disinfection would have to be repeated. If this is more than one filter flushing, which must be performed because of rising differential pressure, or it is even shorter? The distances between the filter rinses can therefore (of course according to the rules) be adjusted. Now to …
Optimization, the Third!
Now if you know, after which time the result is satisfactory, the concentration of the disinfectant can be reduced. In each filter rinse, the concentration of the previous filter rinse is halved until Legionella is detectable in the 15-minute sample. For the previous concentration of the disinfectant then there is sufficient security.
Of course, it is necessary for this measurement, always pause until a second measurement, the Legionella-freedom of the filter is detected. The bathers are finally no subjects.
Optimization for all,
who still want to try it with chlorine. The chlorine kills the amoebae, Legionella therein multiply further - until the cases "amoeba" bursts. Until then, a valley is in the results, in order to rise again faster than the pre-level. An examination of the Legionella population should therefore take into account the rhythm of bursting Amoeba capsules. Experience shows that after 4 Days will be the next disinfection due.
Summary
Perhaps we could organize something the chaos from the beginning. The rules and regulations leave us alone for long stretches. But with "looking over the plate edge" and a little perseverance, there may be a not so expensive way to the same destination. Not always the way is described in a rule and still shorter.
Thank you for the interest! I am glad about every feedback.

♦
Other languages available online only.




Leave a Reply
You must be logged in to post a comment.