Published in: Das Schwimmbad und sein Personal, edition 05/2018
... or how we can keep an eye on our hygiene parameters in the future!
It's been a few days, the last post had, as I hope, quite intensively engaged in the process stage filtering. This post skips the last made recently promised employment with the filter cleaning. Here and now it should therefore go to the measurement and control for disinfection. Mixed with some basics and (m)a hopefully particular view.
Internet of things is literally on everyone's lips, and describes, such as the Internet in equipment has arrived. In our industry, you might think, IOT aviod the pools. We should change that together! Maybe it just needs a new slogan for it, as Industry 2.0 or GroKo (German special for "Big coalition"), in which the name is more powerful than the content. The chemical basis for disinfection have not changed significantly, we will address these fundamentals. How does its own
Internet of water
can be set up and use in each pool, but will play a major role in the following. A colleague from a major publisher said "repetition makes the slogan!“, if someone asks, why title and first apparent title match. Later in this article we look at the content, stuck in there (should).

Transfer of parameters and values via the Internet, Photo 1: aqua&pools
Hygiene and disinfection
To repeat the general textbook wisdom our time together is too good. But on a few basics we need to agree, before we can go into detail. The article deals exclusively with, as optimally filtered water of a swimming- or wellness pool can be disinfected with chlorine. Supplementary methods, such as UV irradiation or ozonation or combinations are to remain excluded here. Similarly, targets outside of the pool water, as the area of disinfection or the disinfection of the activated carbon in the filter. Because of the often completely different notations used I ask for understanding. We simplify this and continuous use even though some companies use a different meaning.
Why disinfection?
we are guided by the general definition: Disinfection speaking at a germ reduction ... by a factor of at least 10−5. This means, that originally 1.000.000 germs capable of reproduction (so-called colony-forming units (KbE, engl. CFU)) not more than 10 to survive.
Well, in the general definition is not, how fast the germ killing must pass. Here is the DIN 19643 more accurate, in which she says: "For disinfecting germ killing of Pseudomonas aeruginosa by four orders of magnitude within 30 seconds was used."
The assumption behind, that if you all of the DIN parameters 19643 comply, the desired concentration of chlorine sufficient, comply with the formulated disinfectant target. But of course the norm-visitors in the standard-number has to jump into the pool.
The manifestations of chlorine, Part 1
The reactivity of chlorine is, that we want to disinfect. At the same time it is this responsiveness, which brings up the chlorine in various forms. This consciousness is the first step in understanding the disinfecting effect.
In DIN 19643, Update 2012, already 5 different chlorine chemicals listed, are added to the water as a disinfectant.
These chlorine chemicals in common, that they oxidizing to the organic and mineral contents of the water bound chlorine or react to form salts. Of course run shortly after the addition and the first side reactions, for example, if chlorine gas to form hydrochloric acid, parallel from. What's left, we call commonly referred to as free chlorine. The sum of free chlorine and bound chlorine becomes Total chlorine listed.
Usually you look at the concentration of free chlorine within a (DPD-) measurement, compares with the nominal value according to DIN 19643 and believes, sufficient to disinfect. But, even if you can correctly measure and interpret the values, is the concentration of free chlorine alone does not guarantee the disinfection performance. There are still some factors at play, the first we have to take.
The partnership of isocyanuric acid and chlorine
Trichlorisocyanuric acid (completed: chlorinated isocyanurates) is an organic chlorine product, the as a depot for free chlorine is working. The advantage of using Trichlorisocyanuric acid, a permanent (Re-) dosage be unnecessary, bought it with considerable measurement errors and -mistakes.
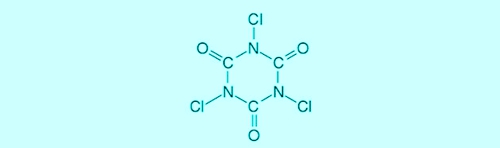
Illustration 1: Structural formula trichloroisocyanuric acid
But instead of clarifying these errors, one could also refer to some of the literature as "disqualification of the pool master". Quote GUV (Rules of the German Social Accident Insurance) information 213-040, Hazardous substances in the treatment of swimming- and pool water, 2015, point 2.1.6: "Trichloroisocyanuric is not as a disinfectant according to DIN 19643 "Treatment of swimming- and pool water listed ", because with this disinfectant no exact chlorine measurement with the required in public pools and existing measurement- and control technology is possible.
One use is in small aquaparks and pools without measurement- and control technology possible. "
Do I understand this in the right manner? Because a swimming pool with measurement- and control features is equipped, using is not allowed? The industry sells these measurement- and control technic but also in areas, where the DIN and GUV be kept for license plates ...
So we come to investigate the mistakes, later it goes to measurement- and control technic, that helps, to avoid these errors.
Imagine a sponge, consisting of Trichlorisocyanuric acid consists. The water decomposes (called hydrolysis) these sponge very slowly, there may be more than 30 take days.

Trichloroisocyanuric acid in water, Photo 2: Fotolia
The sponge disintegrates into hypochlorous acid, which acts as a disinfectant, and in excess isocyanuric. The (chlorine-adjusted, not "trichloro-") Cyanuric acid can be represented in two structural formulas simultaneously. Refer to the diagram 2: Structural formulas of cyanuric acid can be clearly seen, that the chlorine on the right side of the reaction against 3 Hydrogen atoms is replaced.
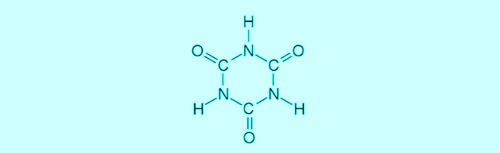
Illustration 2: Structural formulas of cyanuric acid
The Trichlorisocyanuric acid reacts in water to form hypochlorous acid and cyanuric acid. Simplified results in the following reaction:
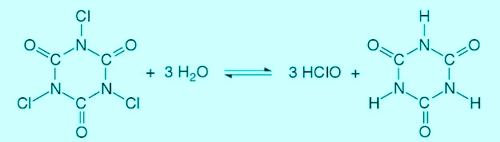
Illustration 3: Reaction of trichloroisocyanuric, Source: Rolf Pohling, Chemical reactions in the water analysis
The dilemma is in the time course. While the hypochlorous acid is consumed in the disinfection, remains "get" the isocyanuric acid and is more concentrated in water. So what happens when using Trichlorisocyanuric acid? Nothing terrible ...
Let's assume, a few days have passed since the dosage and the measurement- and control system has a concentration shown. Now the professional comes to measuring- and control system, pulls out the measurement DPD1, determines the free chlorine and marvels at high values. No.1 mistake is, that DPD1 always free chlorine displays. In truth, it is here the sum of free chlorine and the plug-end more in the sponge chlorine content of Trichlorisocyanuric acid. What to do? Parallel measurement of the concentration of Cyanuric acid and forming the difference between two measured values.
Two devices or one device which can both of? Is there already!
Multi-Parameter-Photometer, Photo 3: Dosatronic GmbH
Quite as simple as in school, it is not. With this knowledge you can the actual content free chlorine calculate, but a conversion table is needed. Tables are available in the literature, for example, this:
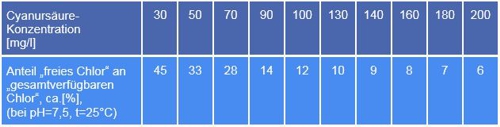
Table 1: Compare isocyanuric, Source Lovibond® Water Testing
Who can calculate, is in a exclusive advantage! Measure the concentration of cyanuric acid and calculate the measurement error! That is expected according to GUV (Rules of the German Statutory Accident Insurance) of the swimming master, but not of the measurement technology?
It is neither more nor less free chlorine in the water, the measurement error or the blindness of the method, which produces the mistake. For this reason, there's also a new name for the chlorine but a name for the measurement error!
If it were not also different, the existing text would have made little sense. Should one not this error compensation in a measuring- and control device integrate? Is there already! Here, this controller is able to this:

Photo 4: Controller with consideration of isocyanuric acid
"Consideration" in the caption does not mean, that the device itself or a connected sensor, Trichlorisocyanuric acid can measure. to enter the measure in the photometer is necessary to set the value manually.
Anyone who has read the previous posts knows, that there must be different methods, than to search in a table and to appreciate. This control is more precise, because a formula is stored here. In the case, the function looks like this:
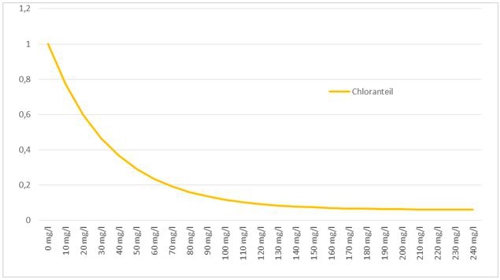
Illustration 4: Measurement errors from the cyanuric acid, aqua&pools
(Intermediate-) evaluate we this knowledge once. If you use cyanuric acid in your water treatment with chlorine and use high-quality amperometric membrane-covered probes, should be careful, that it uses at least one type, is the "cyanuric acid-compatible".
Second, the sensor must be designed so, he existing Trichlorisocyanuric acid (the chlorine depot) next to the free chlorine detected. Of course, a sensor I know, the both requirements are met:

Photo 5: Membrane-covered probe, aqua&pools
Who wants to know the handling, should look for the datasheet following "DOSASens CC1". Of course, a similar sensor can also be found in the program of prominent companies.
Nevertheless, it must be clear: We have spoken so far only through the reduction of errors. The normal tolerances of measurement are still present and add up. So it can only be spoken of a help in estimate the effectiveness. The old method, chlorine dosing easy to block if the pH does not fit, course work in the controller of photo 4 still and can be used in parallel.
The partnership between pH and free chlorine
Who thought, if you now the cyanuric acid measurement error for free chlorine knows, would be enough to evaluate the disinfecting effect, will be disappointed. In part 2 Manifestations of chlorine, we must recognize our powerlessness over chemistry.
If we give chlorine for the purpose of disinfecting the water, we wish, it may be very effective hypochlorous acid HOCl form. But the decision rests solely in the pH-value. Because, after all is dependent on the pH and temperature of a (greater) Part of hypochlorous acid in hypochlorite ion ClO– and hydrogen ion H+ split.
Our only option is therefore, to change the pH-value or to live with a reduced activity. we push our water (on the X axis) Thus, with an acid to the left, the effectiveness increases.
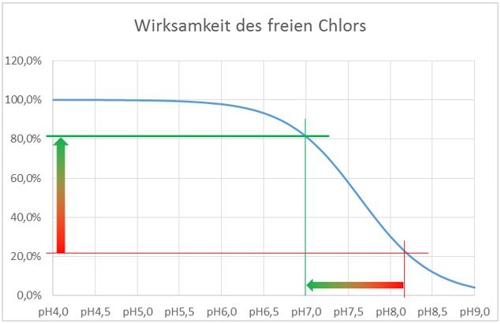
Illustration 5: Representation effectiveness free chlorine
This shift from pH8.2 to pH 7.0 is illustrated by the horizontal arrow and results, that the effectiveness of 21,5% to 81,3% improved. The effect of temperature I have neglected for this example.
Free chlorine means photometer
These relationships are all master of pools known, is described in the literature as dissociation of chlorine in water. But is also known, that a measurement for DPD free chlorine has the same sequence?
The first reagent of a DPD measurement is a buffer, of the sample to a fixed value of approximately. pH6,2 shifts. The coloring, that is made of the second reagent, is defined only in this pH, thus allowing an output of the concentration of the free chlorine.
Pay attention, here you should have write: "of presumably existing disinfectant“. Because, that it is the measured value to free chlorine is about, is only an assumption of the sampler. The DPD measurement does not recognize, if different disinfectants are present.
In addition, the digital display of a measured value with two decimal places in my experience leads to the error, that this result has succeeded "to the point". repeating the (full) Measurement several times with the same water sample will showing , the scattering of the results. It is clearly above the manual says, because the individual fault of the offender be included. What helps? Knowledge, intelligent use and the habit, exclusively by an average of more than 2 measurements to work.
But let's leave it at this point with the limitations of a photometer measurement. It is and remains the best reference method for calibration of all on-line sensors.
Back to the beginnings of the measure free chlorine
For manufacturers of sensors and measurement technology so there is still plenty of potential. The error, with the "smell test" free chlorine to be able to detect, is still widespread in our region. The fact that one does not perceive the free chlorine here but its connection with the urine of the pool neighbor
perceives, is a thoroughly unpopular fact. Let's skip this step!
Yes, they still exist, who want to assess the ORP electrode, the disinfecting effect. And as braces for bad sensor is the measurement also still in DIN. What we're doing really? The ORP potential characterizes the capacity of the water, take up or release electrons. By chlorine (or better by the hypochlorous acid HOCl) or other oxidizing action disinfectant increases the ORP potential.
but a high redox potential also means, that the water contains almost no reducing or assimilable substances. Errors are so programmed, if other oxidizing or reducing substances come into play. Quote Federal Environment Agency: „For water with an iodide content higher than 0.5 mg / l, the value of the redox potential is sufficient to determine experimentally." Who wants or has in mind, can sometimes look in the periodic table, what elements might still so frolicking with iodine in a group and perhaps just as disturbing.
Here are a qualitative comparison, which redox potentials (ORP) which concentration of free chlorine face (could). Note the Federal Environment Agency may based on this simple relationship.
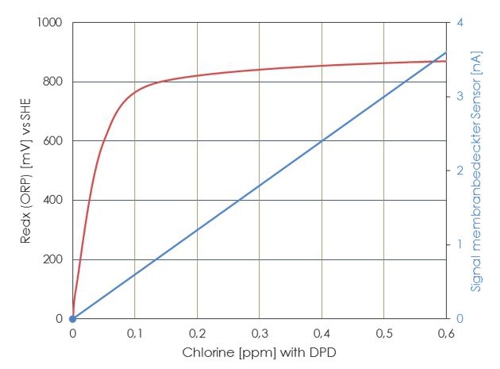
Illustration 6: Waveform redox at opposite DPD reference
It is clearly evident, that a slight shift of a few millivolts in the range 0,3 to 0.6 mg / l can produce a large measurement error.
A redox measurement consists of two electrodes. The reference electrode, mostly made of silver / silver chloride, is located in a saturated potassium chloride solution, and thus has a fixed potential (voltage). The working electrode, usually made of platinum, accepts free electrons from the water. It is an electric voltage of a few electrons forms between the two electrodes. This voltage can be measured, if the meter does not "squandered" the electrons. Devices for are referred to in the electric freaks as "high impedance".
Free chlorine by means of copper-platinum
Do not laugh, it's incredible, still go as many sensors of this type in our southern friends about the proverbial counter. This use is naturally the price owed, you still like to take the inaccuracies in purchase (where the swimmers are not considered as client).
Most metals have a place in the electrochemical series. Here is an excerpt, of copper and platinum shows:
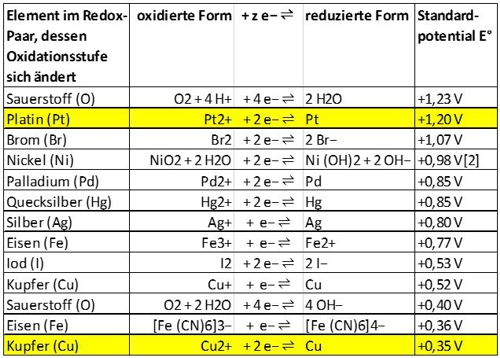
Illustration 7: Excerpt electrochemical series, source Wikipedia
Both metals are in a conductive environment, so that a galvanic cell may form. Das "nobler" metal, so earlier in the series, is platinum and therefore it takes "willing" electrons. OK then, because then a maximum of the copper dissolves and not the expensive platinum. This happens all by itself. The voltage of approx. 850mV, as the difference between + 1.2V and + 0.35V, is supplied from the nature and motivates the lone, which could be supplied by chlorine, to move to the platinum through. It is not the optimum voltage, but "to estimate it's enough".

Illustration 8: Copper-platinum electrode, Photo aqua&pools
While there is an equilibrium reaction, that would come to a standstill after the replacement of the electron, But when you connect the metals electrically, the electrons find their way back to copper. This walk is called clearly electricity flow and measures the few nano-amps. Because this electricity is largely proportional to the concentration of oxidizing substances, he will measured and over a factor as the concentration of free chlorine interpreted. A current is measured, therefore it is called "amperometric measuring system".
Anyone who has read carefully, the noticed: "could be supplied by chlorine", because this probe can (again) not differ, whether the electrons coming from the chlorine or other substances.
noticed, also this contribution is moving back to this scheme of evolution:
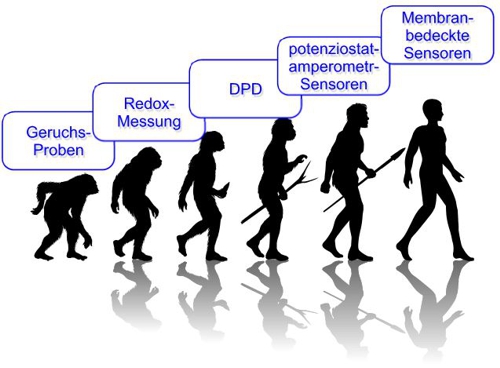
Illustration 9: Evolution of chlorine measurement, aqua&pools
(for automatic translation, left to right: 1-odor samples, 2-Redox (ORP) Measurement, 3-DPD measurement, 4- potentiostatic-amperometric probes, 5-membrane-covered probes)
Free chlorine amperometric sensor by means of potentiostatic-
What the copper can, the experienced measurement technician can do better! At this point, please forgive my literary homage to the German industry specialists Mr. Christoff Scheffold! What can the copper? It can build a natural potential for platinum.
The experienced measurement technician takes the reference from a ORP electrode and replaces the natural potential- or voltage difference to a noble metal surface against an artificial. The electronic component, constituting such a potential difference and constant (static) keeps, called potentiostat. The current will be measured (amperometrisch), which is necessary, to keep constant the voltage.
The experienced measurement technician is usually more intelligent than copper. Therefore, he knows, in which (artificial) which voltage difference disinfectants liked to by his electrodes separated. Therefore, the measurement can be set to the disinfectant much better than copper. At the same time, the measurement of Chlorine dioxide and Ozone possible.
New problem, the same sentence structure
The potential difference is, which we want to have for measurement. At the same time it is also potential difference, the pads can cause on the metal surfaces and thereby complicate the measurement. This problem adds up to a signal change by pH value, Temperature-, turbulence- and flow changes. The experienced measurement technician can compensate for this, we are thus but thematically arrived at complete measurement systems. The appropriate sensor is not enough, to influence these factors.
The simultaneous measurement of pH, Temperature and flow rate, we do not need to explain further, the turbulence can be defined about the environment of the sensor, but how can you deal with the contamination of surfaces?
Here, the construction of a flow fitting, the 5 parameter combines: pH, ORP, disinfectant, Temperature, Water flow. in Figure 11 you will find the real part on the left side.
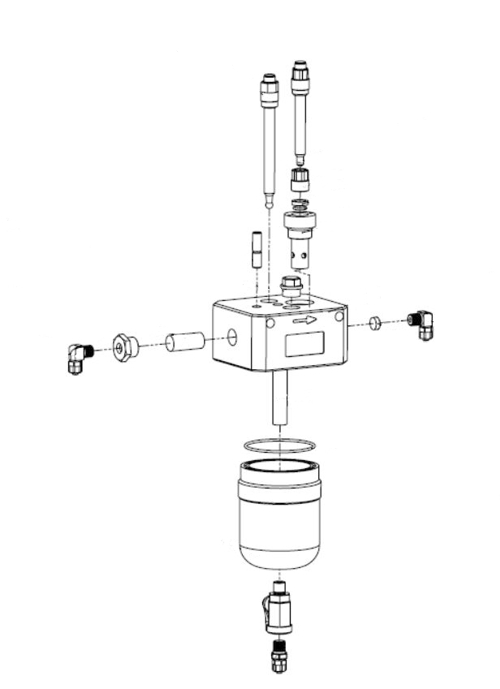
Illustration 10: Exploded sketch from manual to figure 11
OK, Swimming pool water should be more clean. But fingerprints or the biofilm after a few hours without disinfectant play a role.
A method of the aforementioned specialists provides a good solution for my opinion available. The measurement is periodically turned off shortly and generated on the gold surfaces a weak electrolysis. The forming gas bubbles clean the surfaces of the sensor.

Photo 6: Potentiostatic amperometric probe
A practical example of a measurement system, which all 5 gets conditions under control, is this:

Illustration 11: Measuring system DOSACompact, Foto aqua@pools
Another for me better way, to master these limitations, is the ...
Membrane-covered amperometric probe.
What is different? In the name but the potentiostat is missing otherwise at first view only one: An electrolyte-filled cap with a tiny membrane, which is the working- and the reference electrode enveloped. The devil is always in the details, because these little things it contained! Many Far East prospect is failed at these little things. The membrane can be obtained by material, Position and size differentiate the disinfectant. One of membrane types allows gases to pass, the other only electron or liquids. The disinfectant, or what could it pass through the membrane, then must it reaches the working electrode take a small path through the electrolyte before.
Modern membranes are not disturbed by a biofilm on its surface. And if it enters a circumstance, which "blocked" the membrane, a change in the membrane cap is possible. The reference electrode can "swim" with a much larger surface area in the same electrolytes and provide an extremely accurate reference potential for other disinfectants. Unlike the potassium chloride solution used otherwise, the electrolyte added to the sensor is not equal to a death-blow. changing a few simple steps possible.
The built-in temperature compensation is already taking in the sensor fault cause from consideration.
We approach clearly the current end of the evolution of sensors for free chlorine. But on a Basic we have to go back: So far, the sensors have SOMETHING measured, what somewhere between hypochlorous acid HOCl and what we free chlorine understand, located. always, when one turns on the pH-screw, is the signal of the sensor "anywhere else" but not concerning free chlorine. The reason is the direct contact of the working electrode with the hydrogen, its concentration is specified by the pH value.
When membrane-covered sensor, the membrane and the electrolyte protect mostly against the influence of hydrogen. Not completely, and depending on type, but as a known quantity. The changes have been tested and are available as a mathematical formula for each controller available. A chart we save here, a photo to the controller, can use this data and does, also. It is only important: Is there already! See photo 4.
Calibration, a vexed issue
In connection with the DPD measurement we had seen, that it depends on knowledge and handling. What's calibration? It is the setting of a factor in the controller(!), which indicates the proportionality between the signal of the sensor and the displayed value of the controller in the desired unit. For free chlorine would be the factor, sometimes called slope, the ratio of the signal and value.
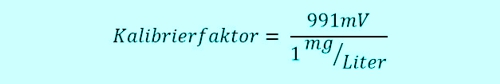
So you do not calibrate the sensor, but the controller! It therefore makes good, if the developers of the regulator have made worrying for calibration. Take an example:
The technical assistant takes 11:00h a sample from the flow-through fitting close to the measuring system, which is currently working on real 0.6 mg / l. Thus, the sensor sends 0,6 x 991mV = 595mV to the Controller. Two groups of children have the swimming pool 10:45h entered, are fresh "sun protected" and jump with joy at 11:05h in the pool. The free chlorine doing his job, oxidized and disinfected so go ahead. The concentration takes 5min long heavily down, that sample water system transports the water in very short 5 minutes to the sensor. The concentration in the pool has now dropped to 0.4 mg / l, the sensor sends 396mV.
Meanwhile, the pool assistant has evaluated the sample by DPD and formed the average. He has worked super well and has come to a concentration of 0,59mg / l. He gives this value 11:15h in the controller. What happens normally? The current signal is 396mV than 0,59mg / l interprets, although only 0.4 mg / l in the pool.
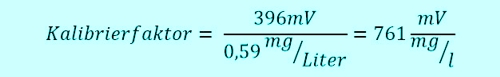
At least until the next calibration the pool will 32% operated with too little free chlorine. What can you do? Write down the value initially 991mV and position the classical relationship equation. Or, you guessed it certainly, using a controller, which remembers the signal value for you. Yes, there's already existing. See photo 4: Controller with consideration of isocyanuric acid. ... because me a waste of time for hundreds of unsuspecting calls, that "everything does not work" with other controllers, has annoyed!
In all fun, that I had personally in writing this article, I have to be fair, again point out you to the following fact: I'm on the editorial board in the BDS and write here as an engineering firm aqua&pools in my spare time. In main job I'm Technical Director of DOSATRONIC GmbH and was able to my ideas "per associacion masters of pools" apply myself.
Technique of the 70's!
Who helps the most modern sensor, if the controller can do nothing with it. Who does not know that? One finds controller in the technical area, which technique could originate from the 70s. A box in the filter room, difficult to access, 2-line display in an attractive green, will show the current measured value and costs nerves, until you memorize the 5-level menu and 4 can operate with all 4 keys. And when a change is necessary, then the now 72-year-old computer programmer from the retirement must be obtained, to connect to a more modern sensor. Wishes, as another language or even the improvement of spelling mistakes take then ever between 3 2 pension payments.
Then we change but most quickly in the age of:
Internet of water.
Everything is now on the Internet, why not the measurement and control of disinfection. Controllers, which spread their data over the Internet, have been available for some years. Many but with the disadvantage, that the informations are routed via an external server. A rogue, who has assumed the fishing out of customer contacts even before the current scandals. In contrast, only helps, to be able to run a web server directly in the device and control its communications directly. developed, implemented!
Another danger in the focus of time are computer viruses. But here our disinfectant does not help. Similar to the last post about filtering applies here, where no host (like a the operating system) since no virus! The work of the internal processor is on his duties, measuring and regulation, limited. Reproduction of "worms" not one of the tasks.
And what, if the processor fails on his duties? Well, then the second processor sounds the alarm. Because he has the tasks of a "watchdog", as also occur in automotive ECUs. implemented!
Up to this point so many features have come together, it with a 2×16-Character display is almost impossible, everything (understandable) to implement. True to the slogan: "Cutting old habits (only complete) down!" now is the display doubted. Why a display, if the data anyway 23:15 Hours are unseen?
Do we want to pay for this service, to go to the basement, to control the observance of values and the level of containers? That may be profitable in North Africa, in Europe it is by no means. That's why: The display had to go!
Each of us is surrounded in most situations of displays, be it on the PC monitor, on the tablet or the smartphone. So why not transferred to these displays, What else get in the basement only the filter to face? A little HTML code inclusive, and the controller displays on each display, what he can do so. You guessed it, there's already existing! Yes, as already said, see photo 4!
How does it work? Well, by simply dialing with a modern current browser to the IP address of the controller and thus has all the possibilities of the controller anywhere with him. Of course, assuming proper authorization. patience, later in the text there is a possibility, to view an example.
If you have a "real" monitor in front, the information is displayed so:
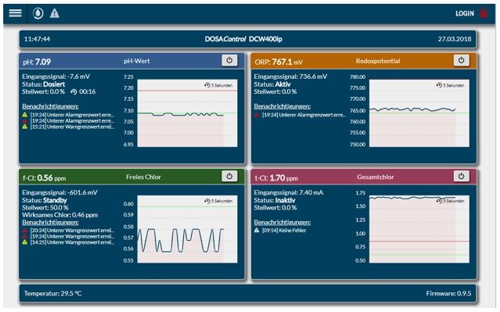
Illustration 12: Screen capture DCW 400IP
The smartphone in landscape mode restricts the information only slightly, because by scroll sideways the other panels are visible.
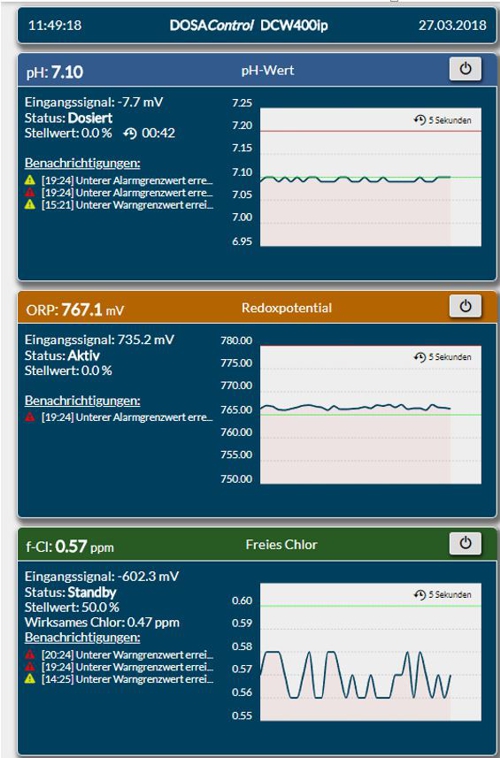
Illustration 13: Screen shot in the smartphone
Particularly small displays only the respective header with the concentration is displayed.
Who is disrupt by the strong fluctuations in the value, the pictures are taken from a simulation. Those who want to get an idea, "Look at" going, "Play around" is also possible, setting something else is not possible! Under the address is a simulation of the surface to find: www.dosatronic.de/dcw400ip/index.htm.
Of course, additional features are also integrated. For the customer pre-stored sensors or simple language change a part of it. You can save the complicated parameterization of the PID controller also ... by activating the adaptive controller, or to configure sending email to, that the end of the chemicals will be communicated directly to the Treasurer. Enough of it, now on the
Internet of water!
Have lots of fun with it! Only those who test, can gain insight. If you work in a group with several swimming pools, you can also bundled the parameters of several pools or swimming pools, For example, in a table, View side by side. Ok, the controller is not now ONLY for pools with classical pH / ORP / free chlorine designed, But in addition to temperature and flow measurements and a fourth channel can equip itself as desired.
Yes, slowly this article should end. You guessed it, it left open a few more topics, which can be useful in everyday life in the measurement. Promised, I'm working on it! Next time it comes to hydraulic systems in the pool. The pool hydraulic philosophers of you are fond invited, to share their experiences in advance by email to me.
Thank you for the interest! I am glad about every feedback.

Free but not for nothing!
Together, BDS and aqua&pools are able for members of the association total 3 Measurement- and control systems DCW 400IP make free available. Members, who want to replace the existing device or your require initial, should focus on
www.aquaandpools.de/Anmeldung/
information and then login. The systems are in pre-assembled by the manufacturer for the measurement of pH, ORP and free chlorine with the necessary fittings provided. A connection to the Internet via local Ethernet is the same as the readiness, to document the experiences, required. (Offer expired!)
The legal process is excluded. ♦
For registered members here the print version as PDF:
[wpdm_package id=’3075′]
Leave a Reply
You must be logged in to post a comment.